
Clinging to her mom, this baby Coquerel’s sifaka represents the only lemur species at the Duke Lemur Center known to fall prey to cryptosporidium, a microscopic parasite that causes diarrhea that can last for a week or more. The illness wipes out much of the animals’ gut microbiome, researchers report, but fecal transplants can help them recover. Photo by David Haring, Duke Lemur Center.
DURHAM, N.C. — “Stool sample collector” is not a glamorous way to introduce oneself at a party. But in the course of their research, gut microbiologists Erin McKenney and Lydia Greene have spent a lot of time waiting for animals to relieve themselves.
They estimate they have hundreds of vials of the stuff, from a dozen primate species including lemurs, baboons and gorillas, sitting in freezers on the Duke University campus.
The researchers aren’t interested in the poop per se, but in the trillions of bacteria inhabiting the gastrointestinal tract, where the bugs help break down food, produce vitamins and prevent infection.
A few years ago, McKenney and Greene started collecting stool samples at the Duke Lemur Center to see how the microbial makeup of lemurs’ guts varies from birth to weaning, and as their diets change over the seasons. And what happens when they get sick?
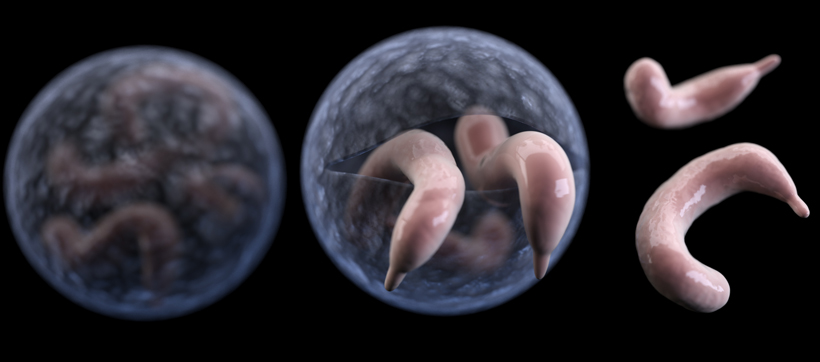
Illustration of Cryptosporidium, a widespread intestinal parasite that causes diarrhea in people, pets, livestock and wildlife worldwide. Courtesy of the U.S. Centers for Disease Control.
Between 2013 and 2016, ten of the lemurs they were studying contracted cryptosporidium, or “crypto” for short, a waterborne parasite that causes diarrhea in people, pets, livestock and wildlife worldwide.
All of the infected animals were Coquerel’s sifakas — the only lemur species out of roughly 20 at the Duke Lemur Center known to fall prey to the parasite — and most of them were under five years old when they fell ill.
Animals that tested positive were moved into separate holding areas away from other animals and visitors. Keepers wore protective suits, gloves, face masks and booties while working in the animals’ enclosures to prevent infection.
All of the animals eventually recovered. Along the way, six of the affected animals were treated with antibiotics, and three were also fed a slurry of saline and feces from a healthy relative.
McKenney and Greene collected stool samples before, during and after infection for up to two months. They used a technique called 16S ribosomal RNA sequencing to identify the types of bacteria in the samples based on their genes, and compared the results with those of 35 unaffected individuals.
In a healthy gut microbiome, “good” bacteria in the gut compete with “bad” microbes for space and nutrients, and secrete substances that inhibit their growth.
The guts of sick and recovering sifakas are host to a very different assortment of microbes than those of unaffected animals, the researchers found.
Not surprisingly, both crypto infection, and antibiotic treatment, wiped out much of the animals’ gut flora — particularly the bacterial groups Bifidobacterium, Akkermansia, Succinivibrio and Lachnospiraceae.
Even after the infections cleared, most animals took another several weeks to stabilize and return to normal levels of gut biodiversity, with younger animals taking longer to recover.
The only animals that made a full comeback within the study period were those that received a fecal transplant, suggesting that the treatment can help restore gut bacterial diversity and speed recovery.
The patterns of gut recolonization following crypto infection mirrored those seen from birth to weaning, said McKenney, now a postdoctoral researcher at North Carolina State University.
The researchers hope their findings will help control and prevent crypto outbreaks in captive primates. Because lemurs are more closely related to humans than lab mice are, the research could also help scientists understand how the gut microbiome protects humans from similar infections and facilitates recovery.
“Thanks to bioinformatics and advances in sequencing, the microbiome gives us a window into the health of these animals that we’ve never had before,” said Greene, a graduate student in ecology at Duke.
They published their findings June 15, 2017, in the journal Microbial Ecology in Health and Disease.
Duke evolutionary anthropology professors Christine Drea and Anne Yoder were senior authors on this study. This research was supported by the National Science Foundation (1455848) and the Duke Lemur Center Directors Fund.
CITATION: “Down for the Count: Cryptosporidium Infection Depletes Gut Microbiota in Coquerel’s Sifakas,” Erin McKenney, Lydia Greene, Christine Drea and Anne Yoder. Microbial Ecology in Health and Disease, June 15, 2017. http://dx.doi.org/10.1080/16512235.2017.1335165

Post by Robin Smith, science writer, Office of News & Communications
